Scientists and clinicians from across the globe shared insights into the therapeutic potential of cannabis in London last week. We explore some of their findings here.
The International Congress on Clinical Trials on Cannabis (CT-CANN23) took place in London on 15–16 February, inviting experts from several continents to showcase their contributions to the growing evidence base for the therapeutic use of cannabis.
The event saw leading scientists, clinicians and industry professionals explore ways of improving current research strategies while discussing new discoveries and future avenues in the rapidly evolving field.
Discussions over the course of the two days focused on clinical methodologies, regulatory issues and pharmacological considerations, as well as advice on the practicalities of prescribing, with support from the Medical Cannabis Clinicians Society (MCCS).
Findings covered a broad spectrum of emerging areas of cannabis research from cancer and chronic pain to anxiety, PTSD and even inflammation associated with tooth loss.
Read on for some of the key insights:
CBD and anxiety: world’s first clinical trial
Cannabis researcher and Harvard University neuropsychiatrist Dr Staci Gruber shared some initial findings from the world’s first clinical trial of a full-spectrum, high-CBD product for anxiety, which she conducted as part of the Marijuana Investigation for Neuroscientific Discovery (MIND) programme.
In this open-label trial, participants were given a dose of oil containing 10mg CBD and 0.26mg THC, three times a day for four weeks. Their outcomes were measured using two standardised self-reporting questionnaires, plus the clinician-administered Hamilton Anxiety Scale (HAM-A).

McLean Hospital Cognitive and Clinical Neuroimaging Core and Marijuana Investigations for Neuroscientific Discovery (MIND), and Dept of Psychiatry at Harvard Medical School, USA
The product was ‘well tolerated’ with no adverse effects or intoxication reported. Analysis revealed ‘significantly improved’ ratings of anxiety, as well as ratings of mood, sleep and quality of life. Dr Gruber also noted ‘stable or improved performance on tasks of executive function’. A double-blind placebo-controlled phase is now underway, alongside clinical trials exploring the effects of CBD on anxiety in patients with glioblastoma and in bipolar disorder.
Observational data collected through the MIND programme, which Dr Gruber founded in 2014, informed the study and has paved the way for several other clinical trials, she explained.
“Each type of study design has strengths and weaknesses and they generally complement each other,” Dr Gruber said.
“We need real-world data, from real-world patients, taking real-world products. These data provide important insights into what specific products could be efficacious for specific conditions and indications, and should be targeted in clinical trials.”
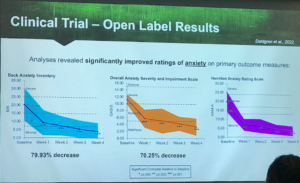
Results from the open-label clinical trial on anxiety and CBD. Credit: Dr Staci Gruber, MIND Programme
Cannabis in dentistry: inflammation and tooth loss
Periodontitis is a severe gum infection that can lead to tooth loss as well as other serious health complications. There is currently no cure, but the disease can be managed through regular maintenance. Researchers based in the Czech Republic have been exploring whether CBD could help reduce the inflammation associated with the condition, alongside its potential benefits and drawbacks in the field of dentistry.
In this placebo-controlled double-blind study, patients were split into two groups and given toothpaste and dental gel – with or without CBD – while a comparison group was treated just with a Corsodyl dental product.
A statistically significant difference was observed between the first two groups, with the authors summarising: “A statistically significant improvement in gingival indices [bleeding caused by severe inflammation] after 56 days of CBD application in patients with diagnosed periodontitis, with no adverse effects, is reported.”
Brain cancer: increasing survival in glioblastoma?
Researchers in Australia have been exploring the effects of medicinal cannabis oil in patients with glioblastoma multiforme (GBM) – one of the most aggressive forms of brain cancer. There is a significant need for new interventions, with only 5% of patients surviving more than five years after their diagnosis.
Scientists at Southern Cross University, led by Dr Janet Schloss, carried out a double-blind RCT on a group of patients aged 18 and over. One group was given a 2.25ml daily dose of oil containing a 1:1 THC (10.35mg) to CBD (10.8mg) ratio, while patients in the second were given daily doses of 1.8ml containing 4:1 THC (27mg) to CBD (6.8mg).

Dr Janet Schloss, National Centre for Naturopathic Medicine at Southern Cross University, Australia
At the end of the 12-week study period, 11% of patients had a reduction in disease, 34% were stable and 16% saw a ‘mild enhancement’ of their condition. Improvements in physical symptoms, sleep and overall ‘contentment with quality of life’ were deemed to be ‘statistically significant’.
The researchers said: “We believe this study provides good evidence that medical cannabis administered to this patient population is safe and well tolerated and can provide symptomatic relief to these patients.
“Our study data suggests that cannabis can be helpful for many symptoms affecting QoL [quality of life]… especially sleep disturbance.”
In a post-trial follow-up, 47% of patients were still alive, with some having survived up to seven-and-a-half years after diagnosis.
Dr Schloss and colleagues concluded: “A single nightly low dose of THC-containing medicinal cannabis was well tolerated in inoperable and recurrent GBM and AA3 patients and significantly improved sleep, functional well-being and QOL.
“Medicinal cannabis may have the potential to increase survival in patients with high-grade gliomas.”
Veterans: PTSD, insomnia and TBI
Dr Celeste Thirwell, director of the Sleep Wake Awareness Programme (SWAP) in Canada, has treated over 6,000 veterans across the country. In her clinic she uses medicinal cannabis and a range of other non-invasive treatments to treat primarily pain, PTSD, insomnia and traumatic brain injury (TBI).
A total of 22 veterans took part in a pilot study to explore the effects of cannabis on these symptoms and its ability to reduce patients’ reliance on the use of multiple pharmaceutical medications (known as polypharmacy).
Patients were using an average daily dose of 6g over a period of more than a year. According to Thirwell, most started with a CBD oil and added THC flower, vapes and other forms of administration, depending on their symptoms and activity throughout the day.
Results showed that after cannabis treatment, the veterans’ scores on a standardised PTSD checklist had reduced from an average of 67.9 to 34.5 (a score of 50 is considered significant for PTSD). They also reported improvements in sleep, anxiety and chronic pain.
The number of daily medications that patients were taking also reduced from an average of eight to an average of three and, as a result, so did the negative side-effects of those medications, which include gastrointestinal issues, brain fog, erectile dysfunction, sedation, increased anxiety and suicide ideation.
Speaking at the conference, Dr Thirwell said: “What I learned from my work with veterans is that we were able to improve their sleep, decrease their PTSD symptoms so they can function… help their TBI symptoms, improve pain management so they become more mobile… and most importantly, we help their personal and family relationships. We’re saving families with the cannabis treatment that we’re using.”
Headache disorders: cluster headaches
Despite some research linking migraine to a deficiency of the endocannabinoid system (ECS), according to Israeli researcher Dr Amnon Monek, to date, there is little high-quality evidence for the use of cannabis to treat this type of headache disorder. Cluster headaches, however, are a different story.
Only around 1.4% of the population experience cluster headaches and they generally affect more males than females.
An attack can last up to 60 minutes, and someone experiencing a flare-up may have up to eight attacks every day, with symptoms including severe pain, tearing, a runny nose and reddening of the eyes. Around 5%-10% of patients will develop chronic cluster headache, where the symptoms will affect them on a daily basis.
Dr Amnon Mosek, The Headache and Facial Pain Clinic, Sourasky Medical Center, Israel
Several years ago, Dr Monek conducted a retrospective study on 18 patients with chronic cluster headaches who had failed to respond to all other medications. The average length of time they had been experiencing symptoms was nine years, with an average of up to six attacks every day.
Patients sourced their own cannabis and consumed an average of 1g a day, with smoking recorded as the preferred mode of administration.
According to Dr Monek, at a follow-up appointment around half of the patients had not responded to the treatment, but those who did had seen almost all of their pain reduced. Most patients had a 50% decrease in pain severity and were ‘highly satisfied’ with the treatment.
Dr Monek concluded: “Overall we are very happy with the use of cannabis in treating cluster headaches.”
The information in this article was taken from presentations at the International Congress on Clinical Trials on Cannabis (CT-CANN23) in London on 15-16 February. You can see the full agenda from the event and contact the organisers here.
- Jazz Pharma to present new research findings on Epidyolex
- Four new medical cannabis studies to catch-up on
- How does THC work in the body? Researchers share new scientific insights
- Mum to help educate Scottish doctors on ‘life-changing’ medical cannabis
- Cannabis and cancer: everything you need to know
The post Five emerging areas of cannabis research – cancer, cluster headaches and tooth loss appeared first on Cannabis Health News.

