New MHRA guidance for the approval of unlicensed cannabis medicines in the UK is recognition of both the health and economic benefits of a domestic industry, say experts.
The Medicines and Healthcare products Regulatory Agency (MHRA) published new guidelines earlier this month to support companies applying for authorisation of unlicensed cannabis-based products.
According to the regulator an increasing number of companies with ‘no previous pharmaceutical experience’ are entering the sector with a view to cultivating, processing and manufacturing cannabis-based products.
This makes the process more complex, as companies wishing to manufacture cannabis-based medicinal products or active pharmaceutical ingredients require authorisation by both MHRA and the Home Office.
The MHRA says it currently isn’t possible to operate both authorisation processes independently because each requires some aspects of the other.
As a result discussions have been held between the two departments to explore how they could provide a ‘joined up’ approach to the approval process to meet the needs of both the industry and the government.
This new approach is outlined in a recent blog post by the MHRA, clearly setting out each stage of the process.
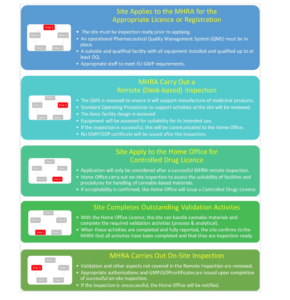
Source: MHRA
The groundwork for a ‘healthy’ domestic industry
The update has been welcomed by some who say the increase in ‘transparency’ will ‘encourage investment’ and ‘lay the groundwork’ for a ‘healthy’ domestic medicinal cannabis industry in the UK.
It also signals government recognition of the potential of the sector to boost the UK’s economic growth and the health of the nation.
Several industry leaders have previously called for a more ‘streamlined’ approach to the licensing process, including the Centre for Medicinal Cannabis (CMC), which highlighted this in its Hodges Review and Decalogue reports earlier this year outlining recommendations for the sector.
In a statement bosses at the CMC said the announcement resolves an issue which has ‘disseminated confusion’ in the industry and ‘prevented the establishment of domestic facilities’.
“This increase in transparency will encourage investment and lay the groundwork to help build a healthy domestic cultivation, production and manufacturing medicinal cannabis industry in the UK,” they said.
“We hope that this announcement signals a government recognition of the growth potential that the cannabis industry holds for the UK post-Brexit, and that we will see further clarifications and calibrations of legislation moving forward as well as a more coordinated approach from the Home Office and Department of Health.
“This will allow the UK to maximise the potential it has to advance scientific discovery and innovation, improve well-being, create jobs and investment in local economies, and enhance the health outcomes of potentially millions of people.”
A ‘significant’ step forward
Melissa Sturgess, chief executive of Ananda Developments, which has a Home Office licence to grow high-THC cannabis for research purposes at its facility in Lincolnshire described it as the ‘most significant step’ for the UK medical cannabis industry since it was legalised in 2018.
She commented: “To see a joined-up approach from the Home Office and the MHRA is extremely welcome, and we believe it demonstrates strong acknowledgement and support for this nascent industry.”
- Study hopes to show cannabis would be ‘cost-effective’ on NHS
- New MHRA guidance on real-world data a “step in the right direction”
- CBD edibles, skincare and oil: We get to know all things Weedibles
- World CBD Awards 2022: Who were the winners?
- Campaigners back calls for government Office of Medicinal Cannabis
The post Is the government finally recognising the potential of a medicinal cannabis industry? appeared first on Cannabis Health News.

